Corrosion on Cars and Caravans: Oxidation vs Galvanic vs Electrolysis
The secret life of three kinds of corrosion eating away at your precious car, 4WD, caravan and exactly what can you do about it in your own FatCave…
If all corrosion ever did was consume caravans and return them to their raw material state of being, like bauxite and iron oxide, I think we'd all be sitting here celebrating.
But corrosion is actually a much more significant problem than that. It impacts you in your workshop or garage, in your car and in all other aspects of life - there's always some corroded thing close by that you might even interact with from time to time.
Let’s look into the trio of mechanisms that commonly cause corrosion and talk about exactly what the hell you can do about it.
The premise for this report sprung from this email:
Purchased a 2015 Jayco Starcraft outback caravan approximately two years ago.
Noticed a short time after the purchase a slight bubble under one of the decals on the side of the aluminum cladding. The bubble got bigger over time and I decided to cut it thinking it was an air bubble and I'd release the air from under the decal. It just seemed like the right thing to do.
A wide powder came out. I understand this to be electrolysis.
Since then the bubble has increased in size, plus I have found two other spots where electrolysis is happening.
- Michael
Firstly, it’s not electrolysis.
Now, if you really do want to deal with this problem, we should drill down into the three mechanisms for corrosion.
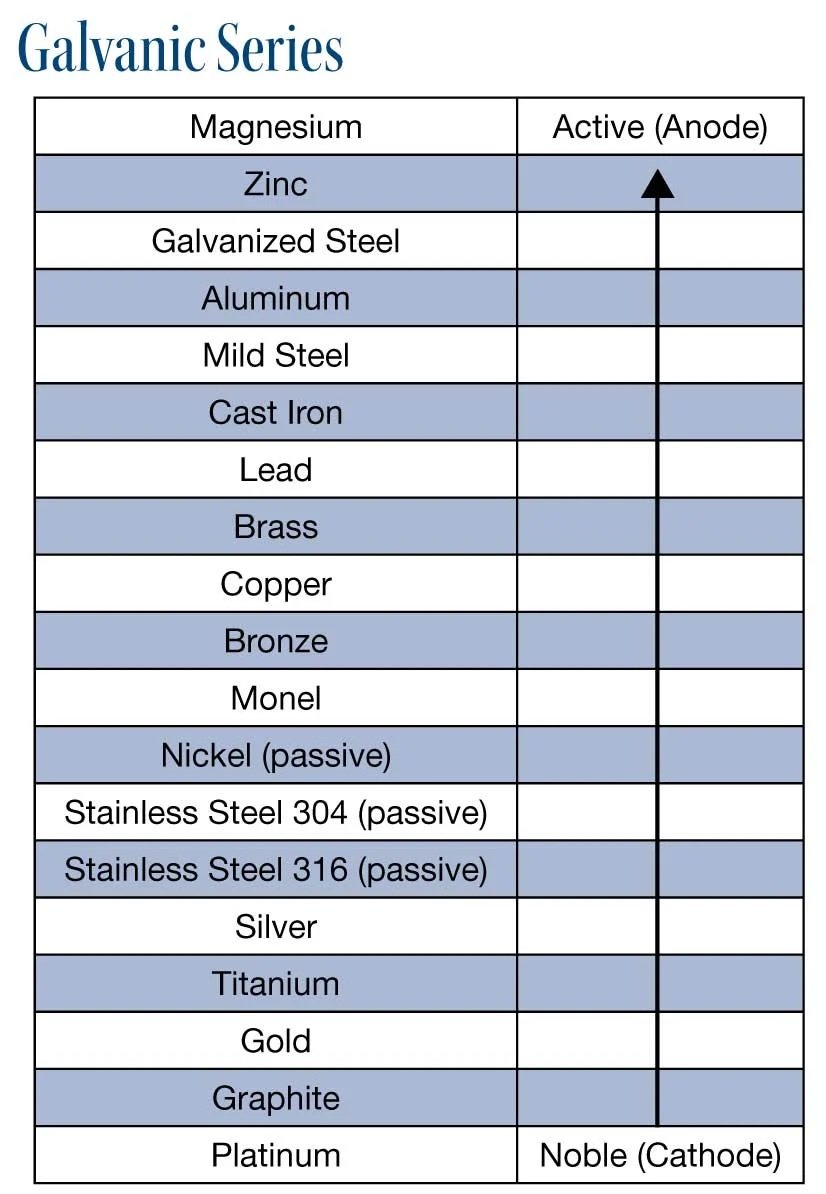
There's just straight old oxidation, which you see all the time. Then there's galvanic corrosion, which is a feature of two different metals in contact with each other in a damp environment, and then there's electrolysis which basically needs a source of electricity.
Beard strokers in the comments sections are very keen to call galvanic corrosion electrolysis, when in fact the corrosion that you generally see on aluminum in automotive-type applications is almost exclusively galvanic.
Oxidation is when, in a quite humid environment, atmospheric oxygen gets into the steel and causes orange rust, which you see everywhere on exposed bits of low carbon structural steel that is not painted or treated in some other way. This is the most common kind of oxidation - and everything oxidizes.
We galvanize this steel so that it doesn’t oxidize, and the way that works is by applying a sacrificial layer which reacts with the air and water from the environment - which is why that outer coating turns grey, because it’s protecting the zinc and steel by reacting to atmospheric oxygen.
In the case of aluminium, a really thin layer of oxide forms on aluminum and turns a gray colour. Basically, that is impervious to more air and water, and it stops the aluminum corroding very much more, and this is probably a few thousand atoms thick.
This really thin coating prevents air and water from getting to the underlying metal, so when aluminum things start to fall apart, it's almost certainly not just oxidation because that's not a failure mechanism for aluminum, in the domain of corrosion. If aluminum starts to fall apart it's almost certainly the second kind of corrosion which is galvanic corrosion.
Galvanic corrosion for non-engineers requires you to use an activity series list for metals. The most common highly active metal that is moderately safe to handle is magnesium, but I wouldn't be doing too much with magnesium in the public domain for corrosion protection, like coating it on steel, because it's flammable. Magnesium is the stuff you light on sparklers for the kids. (Although flammability is lost when you alloy it with aluminum in wheels.)
Sodium explodes if you put it in water, and then there's francium which you wouldn't really want to touch anytime soon. So the most common active metal used for its galvanic properties is zinc because it is highly active - but safe. Yay.
You see zinc protecting the steel in pipes, the steel towers holding up electrical grid cables, and forming the crash barriers keeping you on the highway despite being exposed to the sun, wind, rain, snow and all the bird crap you can conceive.
So why does the zinc protect the steel? When you put an active metal like zinc in close contact with a less active metal like steel, such as on a bolt, it coats the surface to prevent oxygen and water from getting to the steel not just physically, but also because of an electrochemical reaction.
That reaction is not electrolysis. The zinc sacrifices itself to protect the steel because the zinc is more active than the steel. The same thing would happen if you coated steel with aluminum or zinc. This is why every second metal roof in the country is coated with zincalume: it's the zinc + aluminum on top of steel. Steel provides the structural performance, the zinc + aluminum provide the corrosion resistance.
It's most probably a galvanic corrosion that is affecting Michael's caravan because for electrolysis to happen you need actual electricity to be flowing through something in the presence of an electrolyte. In practice, although there is electricity in caravans, it's probably not that - you would probably know about it by now in the form of some low-level electrocution every time you touch the door.
It’s most likely related to some steel in proximity to the aluminum, because if that’s the case, the aluminum will start sacrificing itself to protect the steel.
Look hard enough and you’ll see galvanic reactions everywhere, they are all around you (maybe). Keep watching the full report to stay in Wonderland and see how deep the corrosion rabbit hole goes…
My AutoExpert AFFORDABLE ROADSIDE ASSISTANCE PACKAGE
If you’re sick of paying through the neck for roadside assistance I’ve teamed up with 24/7 to offer AutoExpert readers nationwide roadside assistance from just $69 annually, plus there’s NO JOINING FEE
Full details here >>
AutoExpert DISCOUNT OLIGHT TORCHES
These flashlights are awesome. I carry the Olight Warrior Mini 2 every day - it’s tiny, robust, and super useful in the field or in the workshop. Olight is a terrific supporter of AutoExpert.
Use the code AEJC to get a 12% discount >>
Generators suck! Go off-grid with AutoExpert BLUETTI PORTABLE POWER STATIONS
Need mobile, reliable power? If you’re camping, boating, caravanning or building a dirty big shed in the back paddock, and you need to run a refrigerator, lights, air conditioner, cooking, and/or a bunch of tools - Bluetti has a clean, tidy, robust solution…
Get your AutoExpert free shipping discount here: https://bit.ly/3n62heK


































Just because a ute is cheap, that doesn’t mean it’s worth the money. Is the GWM Cannon more than just a cut-price Ranger wannabe? Can it offer towing, off-roading capability and robust design to compete with the big brand dual-cab utes like Hilux and Triton?