Top 10 Reasons Nitrogen Filled Tyres Are a Scam
Paying to fill your tyres with nitrogen is a scam.
Let’s bust this myth
There are 12.5 million cars on Australian roads. That’s 50 million tyres. They’re wearing out every four years - let’s say - and most tyre retailers are busily dry-humping your leg to get you to spend - $10 per corner - to pump up with a miraculous gas: pure nitrogen. It’s potentially a $100 million consumer scam. And shame on you, for not paying attention at school if you get sucked into this pseudo-scientific vortex of bullshit. Coming up: the top 10 reasons why the nitrogen scam cannot be true.
TRANSCRIPT
This segment is inspired by Jimi James, who comments thus:
"Love the show but you are so wrong on nitrogen being a scam. I have been using it in my car and motorbike for two years now and the main benefit for me is the minimal loss in tyre pressure. I used to check my tyres and top up weekly at inaccurate air pumps. Now it's 4-5 months between checks. Last check I lost only 2psi in my car and 0psi on the bike, that’s after 5 months. At Bob Jane it's $7.50 per tyre for nitrogen and top ups are free for the life of the tyre. I live in northern Australia so I may get more from nitrogen due to the extreme heat. Regardless I will never go back to air." - Jimi James
Jimi, I have no desire to be disrespectful, and I appreciate you watching. But, mate, you’re wrong. Couldn’t be wrong-er, if that’s a word. Your experience is hardly scientific proof. If you’re leaving it five months between checks you obviously don’t value your life very highly. Your experience fails just about every test of the scientific method - and, moreover, it isn’t true because it cannot be true.
The scammers say nitrogen does everything short of cure cancer. Twice the chocolatey flavour; half the calories, plus it prevents tooth decay. (I don’t think they go quite that far.) They say steering, braking, grip and fuel economy are all enhanced. There’s a dramatic reduction in pressure loss. Oxidation of the tyre and corrosion of the wheel are reduced, there are fewer blowouts and lower operating temperatures. These things are either insignificantly true - meaning ‘good luck measuring that’ - or they’re outright bullshit.
REASON 1: WHAT AIR REALLY IS
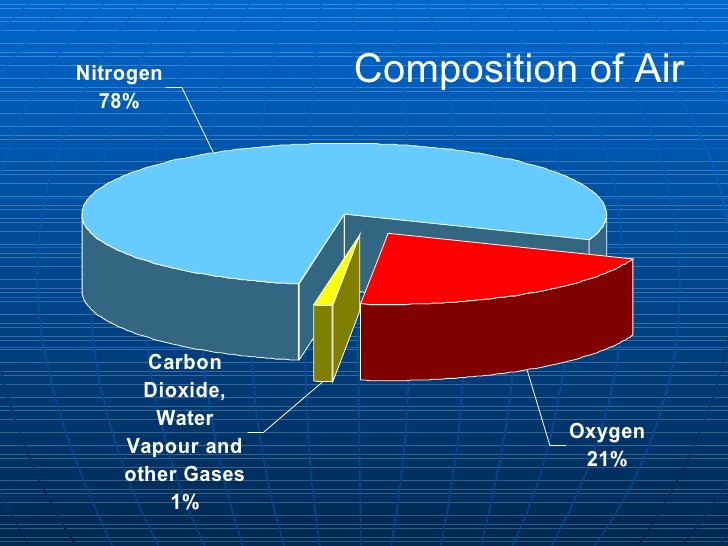
Air - the stuff you’re breathing right now - is 78 per cent nitrogen gas. It’s 21 per cent oxygen gas, and one per cent everything else. So let’s break this down: the difference between pure nitrogen and air is only about one-fifth by absolute volume.
If the 22 per cent of air that’s not nitrogen were completely malignant, plotting the overthrow of your tyres at every opportunity, the Malcolm Turnbull of gasses, the maximum improvement on offer would be 22 per cent. So if you need to top up every week - seven days - on air, then you’d need to top up every nine days on nitrogen.
The losses of pressure you were experiencing previously, Jimmi, were far more likely to be the result of poor tyre fitting, or leaking valves. And how would you even really know at those - quote - “inaccurate” service station pressure pumps?
REASON 2: THE 'LEAKAGE' MYTH
There is a difference in size between nitrogen gas molecules and oxygen gas. Nitrogen is bigger. Bigger is better - and the only people who say size doesn’t matter are people with small gas molecules. Nitrogen retailers (assholes) say it’s therefore harder for nitrogen to escape by permeating its way through the rubber matrix of the tyre - and there is inevitably some gas loss by permeation through that rubber. The assholes don’t drill down into the detail here, though, because doing that would shoot them in the foot.
If you want to measure gas molecules, you need really good eyesight and a tape measure graduated in trillionths of a metre. As it happens, a molecule of nitrogen gas is 300 trillionths of a metre across, and oxygen is 292. So Nitrogen is only 2.7 per cent bigger than oxygen. That’s not much - they might as well be twins. We’re not talking Roseanne and Angelina here…
REASON 3: EMPIRICAL EVIDENCE ON LEAKAGE
There is a slight - insignificant - difference in permeation through the tyre. Respected North American consumer advocacy agency, Consumer Reports, has done a credible nitrogen versus air permeation pressure loss test. They took 31 pairs of tyres, and filled each pair, one with air and its matching other half with nitrogen, all filled to 31 psi and left outside for one year. Here’s what they found:
On average, the nitrogen-filled tyres lost an average of 0.042 psi every week. Air-filled tyres lost 0.067psi every week. So here’s what this means: Every 15 weeks, the air-filled tyres lost one psi, and every 24 weeks, the nitrogen-filled tyres lost one psi. And that means, if you’re losing more pressure than that - you’ve got a pressure integrity problem, not a gas permeation problem. You’ve got a leaky valve, or poor sealing between the tyre and the rim, or poor structural integrity of the tyre or the wheel. Permeation is not a significant cause of pressure loss in tyres.
REASON 4: STEERING, BRAKING & HANDLING
There is no evidence in the entire gamut of applied physics or chemistry that any particular gas is going to produce a tangible benefit to the dynamic performance of a tyre, or reduce the failures. Cornering, braking, steering, rolling resistance, ride quality - they’re all irrelevant to which gas you use.
Pascal - the guy they named the metric unit of pressure after - would be appalled at the suggestion that the type of gas made any difference. Appalled. The gas in a tyre is merely a source of internal pressure, and pressure is just a force exerted at 90 degrees to the inside of the tyre, spread over the area. This is like high-school chemistry. Avogadro, Bernoulli, Pascal, Newton … how hard is it?
Pressure, load, inertial forces … dynamic performance. It’s all completely independent of the flavour of gas you put on board. Any gas will do - within reason. CO2, helium, argon - OK. But if you remember the Hindenberg, it’s probably a good idea not to use hydrogen, or propane, or methane. If there’s a crash, it’s a good idea not to be driving on four flamethrowers… And I wouldn’t be using chlorine or fluorine either. Or Sarin...
REASON 5: OXIDATION
My favourite nitrogen asshole scammer’s claim is that nitrogen is bereft of oxygen and therefore it won’t support corrosion on the inside of the tyre or the wheel. Absolutely true. And absolutely irrelevant and inconsequential, operationally. The inside of the wheel is painted - and I’ve never heard of corrosion on the inside of a steel or alloy wheel being significant in terms of failure or longevity.
Rubber certainly does oxidise, but internal oxidation of tyres is not a factor that affects tyre life. Nobody ever replaces tyres because of internal rubber corrosion. And let’s not forget that the outside of the wheel and the tyre are both subjected, 24/7 to the full 21 per cent corrosive potential of oxygen in air - and they seem to do OK on resisting corrosion.
REASON 6: PRESSURE IN RESPONSE TO HEAT INPUT
Another pseudo-scientific yet bullshit claim is that the pressure will be more constant in terms of heat input. In other words, you’ll get less of a pressure rise in the tyre for any given type of driving if you use nitrogen. Like oxidation, this is technically true, but operationally bullshit. Let’s do what Einstein would call a thought experiment: go to a race track on any particular day, at any particular temperature. And then let’s do a hot lap. A repeatable hot lap. Same speed everywhere, same cornering performance everywhere, same braking performance everywhere. Scientific method.
That hot lap is a source of heat energy going into the tyres. They’re also losing heat as they roll. But there’s a net heat increase because they’re working hard, repeatedly. So the pressure of the gas inside the tyres increases, because temperature is directly related to pressure in the range of operational temperatures and pressures for tyres. Ideal gas laws, if you studied.
Pulling the pants down on this bullshit theory, there’s only four per cent difference in what engineers call the ‘specific heat’ of air versus the specific heat of nitrogen. What this means is: if those tyres run on air and they jump from 20 degrees C from cold inflation to 40 degrees C after a hot lap, and they start at 32 psi, they’ll finish at 34.184 psi. Seems reasonable. Backed up by credible scientific calculations that have stood the test of time since about 1834.
If we replace with nitrogen and go again, same heat input but four per cent fewer degrees in temperature rise. So at the end of the lap, 39.2 degrees C, instead of 40. Pressure at the end of the lap will be 34.096 psi. Less than one tenth of a psi pressure difference. Credible real-world example. Using actual science. If you paid attention at science in high school, you can confirm these numbers.
REASON 7: NITROGEN SAVES WEIGHT
This is my absolute favourite. Nitrogen is lighter than air. Also absolutely true. It is, your worship. Lighter. Measurable lighter. And less weight - especially less unsprung weight - is a benefit to dynamic performance. And tyres are definitely unsprung weight. Another technically true claim that’s brimming with bullshit when you introduce the real world.
In fact, nitrogen is slightly under two per cent lighter than air. Let’s say there’s a volume of 20 litres inside a particular tyre, and you’ve got four of them on the road, so that’s 80 litres. And let’s say you’ve got air at two atmospheres in the tyre … so that’s two atmospheres above one atmosphere (because one atmosphere is a flat tyre - it doesn’t have a vacuum in there, right?). 80 litres of air at three atmospheres weighs about 306 grams. The same amount of nitrogen weighs about 300 grams. The difference - this is for all four tyres - is about six grams. That’s about the same weight as two ping-pong balls. Good luck with the lap record, if you’re counting on nitrogen there.
REASON 8: NITROGEN IS DRY
More practical bullshit here. Nitrogen is often touted as dry or ‘anhydrous’ - and this also is absolutely true. But it doesn’t really matter. Because how wet can air get? If it’s very hot - like Jimmi’s air in northern Australia, at 40 degrees - air can be almost five per cent water vapour. Your 300 grams of air could hold as much as half a ping pong ball worth of water. That’s not a ping pong ball full of water. It’s the same weight as a ping pong ball full of air. Enough for a really short shower. In Lilliput. Of course, if it’s only 20 degrees, air can hold less than five grams of water. A bit over a gram in each tyre. Good luck getting wet with that.
Water vapour in either air or nitrogen does increase the pressure more than a dry gas, when temperature rises. On a road car, the effect is insignificant.
REASON 9: PERFORMS BETTER IN HOT CLIMATES
The heat in northern Australia is extreme, Jimmi - not much fun to live in, at times - but it’s not ‘chemistry lab’ extreme.
Temperature is measured - in chemistry - from the absolute zero temperature point. It doesn’t get colder than absolute zero. That’s minus-273 degrees C (or about -459, I think, in Farenheit). So if it’s 20 degrees in southern Oz and 40 degrees in northern Oz, the temperature difference, in terms of its ability to affect the pressure of gasses and physical things like that is about seven per cent - and pretty much all gasses are affected equally.
Nitrogen and air both react the same to temperatures like that. So it’s impossible to prosecute a case for nitrogen over air just because you live in a warm climate.
REASON 10: MOTIVATION NOT TO CHECK PRESSURES REGULARLY
Up until now, nitrogen was in the category of: It won’t help, but it can’t hurt. But, of course, it does hurt: The biggest problem with nitrogen is the one they never mention. The bullshit pseudo-scientific claims, grossly overstated by the assholes peddling it for their own commercial benefit, is that it motivates people not to check their tyre pressures regularly.
There’s quite a few problems with that. Such as: you pick up a nail in the tread. The tyre starts slowly to go flat. You’re driving down the highway. It blows out (because high-speed running underinflated is the main cause of blowouts). You veer into an oncoming truck. That’s bad.
But if you checked the pressure before setting off, you might notice three tyres at 32 psi and one at 18 - and it might motivate you to enquire why. And save your neck. (Bear in mind you generally can’t see serious under-inflation in a radial tyre - you need a pressure gauge. Or strange mental powers. Or onboard tyre pressure monitoring.)
Even if that disaster blowout doesn’t happen, wear rates skyrocket when a tyre is even a little under-inflated. And, if you’ve got one under-inflated tyre, good luck executing a stable emergency stop or swerve-avoid-recover manoeuver around a child or one of those vermin from the Coat of Arms. It’s just not gunna happen safely, because grip is the other big casualty of underinflation.
CONCLUSION
These nitrogen advocating mongrels are not selling you nitrogen because it’s a tangible benefit to you. It’s not, because a bunch of scientific luminaries who built the technical world we enjoy and take for granted tell us it can’t be. (If you listen.) This is not a matter of opinion - it’s a matter of scientific fact, or fantasy. You can believe nitrogen works if you want, but that view is up there with the sun rising in the west tomorrow morning.
Nitrogen is not a benefit to you. It’s a benefit to the shitheads selling it. It’s a quick cash injection up front, on top of those new tyres - and it keeps you coming back to the same retailer, for more. Neat marketing, huh? The last thing these nitrogen-snake-oil-selling mongrels want is for you to buy your next tyres elsewhere. Peddling this crap is a way of getting you back again and again. It’s relationship building, and it’s a house built on foundations of intelligence-insulting bullshit.